> Introduction
> Sampler Types
· Sediments and Pore Water
· Samplers for Collecting Pore Water or Pore Water—Surface Water Flux
> References

Introduction
This report is a survey of the equipment that can be used to collect sediment and pore water
samples. The report is not meant to be a "how to" on sampler use or sediment site
characterization planning, but rather a basic reference for screening methods for further
investigation. The sediment collection information is mainly taken from Methods for Collection,
Storage, and Manipulation of Sediments for Chemical and Toxicological Analyses (USEPA,
2001) and Assessing Aquatic Ecosystems Using Pore Waters and Sediment Chemistry (Burton,
1998).
The physical location of the sediment, its particle size distribution and compaction, and the final
use of the data often dictate the type of sampler chosen. Physical location considerations include
the depth of the water body overlying the sediment and the strength of the current present.
Unless the sampling event is to occur in a very shallow environment, a bathymetric survey,
conducted prior to choosing the sampling equipment is recommended, and a general
understanding of the current to be encountered needs be obtained. Particle size distribution and
compaction generally dictate whether a given sampling device is capable of obtaining a sample
of the target sediment. Coring devices are usually not effective in gravelly bottoms, and grab
samplers may have problems in areas where there is extensive vegetative debris or compacted
sediment.
The data quality objectives established for the project determine the depth horizon needed for
the sediment sample, the volume required, and the acceptable degree of disturbance. For
investigations concerned with recent contamination events or the affects of contaminated
sediments on the benthic community, the sampling horizon is generally in the 10 to 15 cm range
(EPA, 2001). On the other hand, if historical deposition patterns are the focus or the actual
thickness of contaminated sediment is needed for remedial evaluations, then the required depth
may extend to several meters or more.
The type of chemical or toxicological testing that needs to be performed influences both the
required volume of sediments and the amount of disturbance that can be tolerated. A full
chemical suite of analytical testing for the presence of contamination requires a large volume of
sediments which will result in some degree of disturbance. When the concern is bioavailability,
a large quantity of sediments may be required for testing while preserving the in situ redox
conditions to the extent possible (EPA, 2001). Preserving redox conditions requires maintaining
the sample's integrity by minimizing disturbances in the sediment's structure and exposures to
conditions (atmospheric oxygen) that might change the chemical balance (EPA, 2001). Also the
materials the sampler is constructed of needs to be evaluated to determine if they will have any
impact on the chemical integrity of the sample. Table 1 presents typical volume requirements for
various tests. Tables 2 and 3 provide sample volumes for corers and grab samplers respectively
and identifies the advantages and disadvantages of the more commonly used samplers.
The bioavailability of chemicals in sediments is often estimated using sediment pore water
(Burton, 1998). Pore water can be obtained by ex situ (centrifuge, suction, or pressure) methods
or in situ (probe pumping or diffusion) methods. While in situ methods generally are better than
ex situ at preserving the samples integrity, logistical constraints such as the depth of the water or
the volume of sample required sometimes leave ex situ methods as the only viable choice. If ex
situ methods are needed, centrifugation is the preferred method (USEPA, 2001). This report
does not address ex-situ pore water extraction techniques.
Sampler Types
In addition to the physical conditions at a site (water depth, sediment type, current strength), the
choice of sampler from among the wide variety available depends on what the data objectives
are (e.g., undisturbed core to determine sedimentation history, maintenance of sample redox
conditions, sample analysis volume requirements). Sampler descriptions may be divided into
two large groups: those capable of providing sediment solids and pore water and those capable
of collecting pore water alone.
Sediments and Pore Water
Dredge and Grab Samplers
Although similar in mechanical design to grab samplers, dredges are generally designed to
efficiently remove bottom sediments with little regard for maintaining the integrity of the
sediment. The bucket and dipper designs are examples of these. In the bucket design (e.g.
clamshell and orange peel), the device is dropped into the sediment with its jaws open. After
penetrating the sediment, the jaws are closed and the bucket of sediment is raised to the surface.
Newer designs make the closed bucket water proof so potentially contaminated water does not
drain out the bottom. However, these designs can cause considerable disturbance of the
sediment stratigraphy, and washout of surficial materials is common. The dipper design
resembles the surface operating steam shovel where a rigid bucket is driven into the sediment in
a scooping motion before bringing the sediment to the surface. This design also is subject to
severe washout problems. Dredge samplers are generally not recommended for environmental
sampling, but may be useful in benthic collection (USEPA, 2001).
Grab samplers are designed to minimize the bow wave caused by the sampler's descent. They
typically do this by incorporating flaps on the top of the sampler that open as the sampler moves
down to allow water to pass through rather than being
pushed ahead. Also, unlike the dredge equipment, grab
samplers are designed to minimize disturbance of the
sediment when the sample is taken and brought to the
surface. The flaps mentioned above are closed during
ascent to protect the surface of the sample and prevent
washout.
 Figure 1. Birge-Ekman grab sampler.
Figure 1. Birge-Ekman grab sampler.
Source: USEPA, 2001.
Birge-Ekman style grabs (Figure 1) vary in size with
larger models requiring a winch for operation. The
spring-tensioned jaws are mounted on pivot points and
are set with a trigger assembly that is activated from the
surface by weighted messenger. Flaps on the top of the sampler open during descent to allow
water to flow freely through and close during ascent to reduce the loss of sample. The sediment
can be subsampled through the flaps. Birge-Ekman samplers are suitable for collecting, soft,
fine-grained sediments. Larger matrices (gravel, shells) and vegetative matter tend to prevent
the jaws from fully closing, which results in sample loss and the need to resample (Resources
Inventory Committee, 1998). Birge-Ekman samplers may be restricted to low current situations
and have been known to lose fine surface sediments during retrieval.
Petersen grab samplers (Figure 2) consist of a pair of
weighted, semi-cylindrical jaws that are held open by a
catch bar. The impact with the sediment loosens the
tension on the catch bar allowing the jaws to close.
Additional weights can be added to the jaws to provide
better penetration into harder compacted sediment. As
there is no access through the top of the sampler, only
bulk samples can be taken. Petersen samplers are suitable
for collection of hard bottom material such as sand, marl,
gravel, and firm clay (Resources Inventory Committee,
1998). These samplers are restricted to low current
conditions and may produce a bow/shock wave that
disturbs fine grained sediments. In the presence of
cobbles or vegatative debris the jaws may not completely
close.
Ponar grab samplers (Figure 3) come in two sizes
(standard and petite) and have a pair of weighted, tapered
jaws that are held open by a catch bar. The sampler is
triggered by impact with the sediment bottom. The upper
portion of the sediment jaws is covered with a mesh
screen that allows water to freely flow during descent
thereby reducing the bow wave that precedes the sampler
and reduces disturbance of the sediment surface. Upon
recovery, the wire mesh can be removed to allow
subsampling. Ponar grabs can sample fine-grained to
coarse materials (Resources Inventory Committee 1998).
The standard sampler is heavy and requires a winch for
deployment while the 1 liter petite may not penetrate the
sediment to the desired depth and may require multiple deployments to obtain sufficient
sediment sample. Both samplers are subject to incomplete closure and loss of sample in large
grained sediments or those with vegetative matter.
Shipek grab samplers (Figure 4) have a top cast half
cylinder barrel attached to a lowering wire with
stabilizing bars to keep the sampler vertical. Within
this barrel is a second sampling cylinder that is
activated by a high torque spring. When activated, the
second cylinder rotates 180 degrees through the
sediment and forms a seal with the upper cylinder. The
sample is removed from the sampler by disconnecting it
from the upper assembly. The sampler is designed for
unconsolidated sediments in deep lakes and near
offshore locations to a depth of approximately 10 cm.
Hard, compacted sediments can present sampling
problems and washout of fines can occur during the
ascent of the sampler from the bottom have been
reported with some designs. Large objects such as pieces of wood or shells can be trapped as the
sampler closes causing washout when it is drawn to the surface.
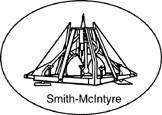 Figure 5. Smith-McIntyre grab sampler.
Figure 5. Smith-McIntyre grab sampler.
Source: USEPA, 2001.
Smith-McIntyre grab samplers (Figure 5) are mounted on
steel frames that can be weighted and ensure the sampler
remains vertical. The two spring-loaded jaws are released
when the frame comes to rest on the bottom. The jaw
tops are covered with brass screens and rubber flaps to
minimize the bow wave on the descent and prevent
sample washout on the ascent. The sediment sample can
be subsampled from the top of the sampler. The typical
sampled area is about 31 cm by 31 cm square. Smith-McIntyre samplers can sample soft, fine-grained to sandy
sediments and are designed primarily for deployment in
marine environments. The sampler requires a power
winch to deploy.
Van Veen grab samplers (Figure 6) are manufactured in
several sizes. A stainless steel screen with rubber flaps covers
the top of the jaws. This design allows the sampler to be
lowered to the bottom with a minimum bow wave, thus
preserving the integrity of the sediment surface. Upon
reaching the bottom the tension in the lowering wire slackens,
releasing the small chains holding the jaws open. Pulling up
on the lowering chain engages the chains attached to the jaw
arms, causing them to bite into the sediments and close.
Latches on the jaws ensure they stay closed. The sediment
sample may be subsampled through the removal of the screens
on the jaws. Lead weights are available to improve the
sampler's penetration into the sediment. This sampler is effective in fine-grained to sandy
sediments that are in deep water and strong currents. The sampler may not close completely
resulting in loss of sediments and requires a winch to deploy.
Hand Corers (Figure 7) are generally suitable for collecting
sediment samples in marshes, streams, and shallow rivers, or at
some depth by diver. Depending upon the sediment composition,
the samples typically are less than 1 meter in depth. Samplers
need to be equipped with a top valve that allows water to pass
through when set in the sediment and closes during withdrawal to
prevent washout. An alternative design is a piston type device that
forms a seal with the corer walls and is drawn or pushed up as the
sample is collected. The piston maintains a vacuum against the
top of the sediment which aids in its retention and prevents water
from entering the sampler during withdrawal.
Russian Peat Borers (Figure 8) are
another form of hand corer that are
side filling and are designed to
collect relatively uncompressed
sediment samples. The components
of the borer include a stainless steel,
chambered core tube; extension
rods, a stainless steel turning
handle; and a core head and bottom
point that support a stainless steel
cover plate. The cover plate is
curved and sharpened to minimize
disturbance when the sampler is
driven into the sediment. Once
driven to the target depth, the core
tube is rotated clockwise to fill the
tube by cutting out a segment of
sediment. The borer is capable of
obtaining samples at depths of 10
feet or more with little sample loss
(USEPA, 1999).
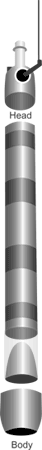 Figure 9. Ogeechee™ sand corer.
Figure 9. Ogeechee™ sand corer.
Adapted from Wildlife Supply
Company webpage.
Ogeechee™ Sand Corers are another form of hand
corer that have been designed to specifically sample
sandy sediments. The corer consists of a core head
that contains a check valve that can be manually
closed by the operator, a stainless steel core body
with plastic liner and core catcher, and driving tip
(Figure 9). The sampler can be twisted or hammered
into the sediment. Extension handles allow for
sampling in deeper water (15 feet) and it can be
used in fast moving water that can adversely affect
the performance of gravity type corers.
Alpine gravity corers (Figure 10) are finless with a
heavy (45 kg, 100 lb) lead weight attached at the
top. The core tube ID is 4.1 cm and can be up to 1.8
m (6 ft) long. A valve at the top of the sampling
tube is maintained by a light spring that allows the
valve to open during descent and close after the
sampler penetrates the sediment. The closed valve
protects the sample from washout during ascent.
Mudroch and MacKnight, 1994, report that this
sampler may have problems entering the sediments
vertically. Also, examination of the cores showed
sheared laminae and disturbed surfaces of the
sediment samples.
Benthos gravity corers (Figure 11) weigh approximately
25 kg (55 lbs) and with extra lead weights require a
winch or crane to deploy. The core tube ID is 6.6 cm and
the upper section has been equipped with fins to aid in
vertical descent. The core tube can recover up to 3 m of
sediment. A removable valve system, located at the top
of the core liner, allows water to pass through during
descent. The valve closes against a machined seat when
the retrieval process is begun to prevent wash out. May
compact the sediment sample.
Boomerang corers (Figure 12) are free falling samplers
that weigh approximately 85 kg and are deployed directly
from the side of a boat. They utilize a disposable ballast
section (nose cone, pilot weight, core barrel, weights,
float release mechanism) and a retrievable float section
(two glass spheres tethered to a core assembly). The core
assembly consists of a 1.2 m by 6.7 cm ID clear plastic
liner with a stainless steel catcher and top cover valve.
After the corer strikes the sediment surface, the glass
spheres are released, and they pull the liner from the core
tube and float to the surface. Sampling depths of up to
9,000 m are possible (Mudroch and MacKnight, 1994).
Box corers (Figure 13) are rectangular gravity corers that
come in a variety of sizes. They can take large relatively
undisturbed samples in soft sediment and are excellent
for sediment water interface studies. There are two basic
designs: an Ekman type where two bottom flaps can be
triggered and the jars close much like the Ekman grab
sampler and the Reinecke design where a shovel like
device slides across the base of the corer. In general,
these corers are large and
can only be operated from
a boat with a large lifting
capacity (2-3,000 kg) and
sufficient deck space to
accommodate it (Mudroch
and MacKnight, 1994).
Piston corers (Figure 14) are capable of
taking cores up to 20 m long. They
generally consist of stabilizer fins, weighted
head, core barrel, piston, core retainer,
cutting head, and trigger mechanism, and
they are deployed by a boat equipped with a
crane. The corer is not allowed to free fall
from the surface. A pilot weight or corer is
attached to the release mechanism by wire.
The length of the wire determines when the
corer is released and the distance it falls.
Piston corers are generally employed for
sediment studies in oceans and large lakes.
Various authors (Mudroch and MacKnight,
1994) report problems with shortened
samples and disturbed/missing surficial (up
to 1 m) sediments when using piston corers.
Phleger corers (Figure 15) weigh about 8 kg
(17 lbs) without additional lead weights and
have a core tube ID of 3.5 cm (1.2 inches).
The top part of the corer has fins for
stabilization and an area for adding weights
to increase penetration. A valve assembly at
the top of the coring tube consists of a
tapered bung that can slide in two
directions—up during descent to allow water
to flow through thereby decreasing the bow
wave and down during ascent to form a seal
on the tapered tube seating and thus prevent
washout and aid in sample retention. This
sampler is generally deployed from a boat to
sample soft to sandy sediments and
semicompacted material in shallow lakes or
marshes. The small sample size can be an
issue when chemical or biological analyses
require larger volumes.
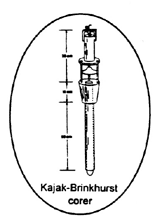 Figure 16. Kajak Brinkhurst corer.
Figure 16. Kajak Brinkhurst corer.
Source: USEPA, 2001.
Standard Kajak-Brinkhurst corers (Figure 16)
weigh about 9 kg (19.8 lb) without additional lead
weights and have a core tube ID of 5 cm (2 in).
Unrestricted water flow through the sampler during
descent minimizes bow wave affects. A valve
located at the top of the sampling tube closes during
ascent to prevent washout. The sampler is suitable
for taking soft, fine-grained sediment samples to a
maximum depth of about 70 cm. While the
standard Kajak-Brinkhurst corer is hand deployable
from a boat, heavier versions may require a winch.
 Figure 17. Vibracorer.
Figure 17. Vibracorer.
Source: USEPA, 2001, courtesy Allen Burton.
Vibratory corers (Figure 17) have a mechanical
vibrator head located at the top end of a coring
barrel. The vibrating head can be powered using
several methods such as hydraulic, electric, or
pneumatic. Different heads use different
combinations of amplitudes and frequencies that
can drive the core tube into the sediment using
primarily a vertical vibration or a combination of
vertical and horizontal vibrations. The type of drive
motion needs to be matched to the expected
sediment type. Vibratory corers come in a variety
of sizes ranging from hand held types to those
requiring hoists. The larger devices can typically
drive a 141 mm (5.56 in) diameter core up to 6 m or
more into the sediment and are generally equipped
with a submersible frame for stability. Operational
depths range from sampling tidal flats to depths of
over 1,000 m (3,281 ft). If deployment is by boat,
an on-board hoist with sufficient lift capacity to pull
the core out of the sediment and enough height to
lift the entire core line out of the water for core retrieval is recommended.
Samplers for Collecting Pore Water or Pore Water—Surface Water Flux
As with sampling the sediment solids, the objective of the investigation determines the best tool
for collecting the sample. The following are potential objectives for sampling water in the
sediments or at their surface to measure contaminant flux.
- Estimate contaminant flux from the sediments into the overlying surface water.
- Establish the presence of contaminated pore water in the sediments (does not require
preservation of in situ redox conditions).
- Determine the concentrations of contaminants that are actually in the pore water within
the biologically active surficial layer of sediments (requires preservation of in situ redox
conditions).
- Perform biotoxicity tests using water taken from the biologically active layer of the
sediments.
- Determine if there is a concentration gradient of contaminants within the sediments.
- Determine flux and composition within the sediments when the surface water is gaining
and a ground-water contaminant plume is present. This determination might also try to
locate preferential pathways into the surface water from the sediment bed.
- Determine the general flux of contaminants into ground water when the surface water is
losing and the sediments are contaminated.
The samplers described below generally have specific uses and care needs to be taken in
choosing the appropriate one to meet sampling data objectives. For example there are several
passive vapor diffusion samplers. One type employs equilibrium partitioning of chemicals
between the pore water surrounding the sampler and the air in the sampler. Another type
employs a chemical trap such as charcoal. The concentration levels found in the equilibrium
device could be very different than that found in a similarly deployed trap device.
Diffusion
Diffusion samplers use a permeable membrane or gel that allows various chemicals to establish
an equilibrium between the water immediately surrounding them and their capture device. The
capture device may be enclosed air, water of an appropriate quality, the gel itself, an ion
exchange surface, organic hydrophobic chemical, or an activated carbon mixture. The
membrane can be chosen to include or exclude various analytes as needed by the project.
Cellulose based materials are not recommended as they tend to biofoul and degrade.
 Figure 18. Dialysis bag on perforated tube.
Figure 18. Dialysis bag on perforated tube.
Courtesy: USGS.
Water diffusion (dialysis) bags are made from permeable dialysis materials (e.g., polyvinylidene
fluoride, polycarbonate) and can be constructed in several designs. Figure 18 shows a dialysis
bag over a supporting perforated frame and within a PVC outer protective shell. They are filled
with water of specified quality and placed into the sediment at the depth to be sampled. This
placement allows them to collect a contaminant
profile of the pore water at specified depth. The
type of water used will depend on the purpose of
the sampling and the ambient water quality. For
sampling water with a relatively high oxygen
content or when a change in redox conditions will
not subvert the sampling objective, deionized
organic free water may be used. If the sediment
body is anoxic and it is important to preserve this
condition in the sampled water, then preparation
of the bag water will have to remove oxygen (e.g.,
nitrogen purging) from it prior to deployment and
keep it from re-entering during transport, handling,
placement, and collection. For redox preservation
the bag and protective cover material should not
contribute oxygen to the water or surrounding sediments. Some plastics have been shown to
diffuse oxygen (USEPA, 2001). Finally, using water with a similar salinity or hardness might be
important to obtaining the sampling measurement objectives.
Peepers (Figure 19) are samplers that employ a rigid body with an opening or openings that are
covered with a permeable membrane or mesh. Acrylic cylindrical chambers are a common type
that contain holes in their sides that are fitted with the membrane or mesh material. Before
deployment they are filled with an appropriate grade of water as discussed in the diffusion bag
section. The cylinders can be deployed in several fashions. For example, they can be stacked in
a specially designed corer so that they sample discrete depths or they can be placed in a shallow
rectangular array for near surface areal distribution determinations. Another peeper design
resembles a box corer with individual cells inside that can obtain a small transect with depth.
The equilibration time for peepers can range from hours to a month depending upon the
contaminant of interest, sediment type, peeper volume, and membrane pore size (EPA, 2001).
Their principal drawback is that they provide small sample volumes.
Diffusion Equilibration in Thin Films(DET) are comparable with peeper systems except that
the diffusive equilibrium is attained between solutes in the pore water and a thin film of gel.
The thinness of the gel (≤ 1 mm) results in faster diffusive equilibration than with traditional
peepers. It has been used to measure Ca+2, Mg+2, Na+, K+, Fe+2/3, Mn+2, Cl-, SO4-, NO3-,
alkalinity, and total CO2 in pore waters at a resolution of 1-2 mm (Fones et al., 2001) and can be
used to measure trace metals.
Vapor diffusion samplers (Figure 20)
are used to sample volatile organic
contaminants by taking advantage of the
concentration gradient that exists
between the contaminants in the
sediment pore water and the air in the
diffusion bag. In one deployment
method, an uncapped 40 ml vial is
placed inside a thin polyethylene bag
which in turn is placed in another
polyethylene bag. The bagged bottle is
placed at the prespecified sample depth
and buried. At retrieval, the outside bag
is removed and a septum cap is screwed
on without removing the original bag.
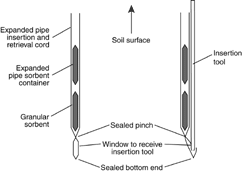 Figure 21. GORE-SORBER® passive diffusion sampler.
Figure 21. GORE-SORBER® passive diffusion sampler.
Source: USEPA, 1998.
In a different arrangement, an open
ended container with a chemical trap is
placed in a polyethylene bag and buried.
The trap (e.g., an activated charcoal formulation) is retrieved and the volatiles are desorbed and
measured at a laboratory. The GORE-SORBER® sampler (Figure 21) is an example of this
design.
Semipermeable membrane devices are plastic bags that contain a hydrophobic organic chemical
that is used to collect trace hydrophobic chemicals in sediment and water (Figure 22). The
protected bag is deployed in the sediment for
a specified period of time. Hydrophobic
chemicals such as dioxins, PCBs, and
polynuclear aromatic hydrocarbons migrate
across the bag and become attached to the
organic fluid contained within it (The USGS
typically uses lipids like triolein or other oil
as the organic fluid). The bag is
subsequently retrieved and the fluid is
processed and tested for these trace
contaminants.
Femto and nano gram/L
detection levels are routinely achieved. Since
the device captures chemicals rather than
establishing an equilibrium between the oil
and water concentrations, the results are an
average encountered over the period of time deployed.
http://wwwaux.cerc.cr.usgs.gov/SPMD/index.htm
Direct Pore Water Sampling
Small diameter piezometers can be placed in sediments where the water is shallow and the
current is relatively weak. When clustered, they can measure hydraulic head differences and
provide pore water samples from different depths for contaminant concentration profiling. The
pumping can be done using suction lift (peristaltic) or, if the inside diameter of the piezometer is
sufficiently wide, a centrifugal or bladder pump. Note that suction lift always applies a pressure
differential across a sample and could affect the analytical results (e.g., negative bias on
dissolved gases and volatile organics). The pumping needs to be done at a very low rate to avoid
mixing of the zones of interest. Piezometers offer advantages over diffusion samplers in that
they can be sampled repeatedly, and they generally do not have volume limitations. On the other
hand, preservation of in situ redox conditions is generally not possible; however, in-line
measurements of parameters such as dissolved oxygen and redox can be made to determine
their approximate in situ values when suction lift is not used.
Syringes can be used to take samples of pore water at different depths in the sediment profile.
They can be purchased with various barrel volumes with a range of needle lengths and bore
inner diameters. Pore water samples are obtained by pushing the needle to the desired depth and
retracting the plunger. Syringes may not be effective in compacted sediments or gravels and can
become plugged in very fine sediments.
 Figure 23. BAT™ sampler.
Figure 23. BAT™ sampler.
The BAT™ system (Figure )is generally associated with ground-water
point sampling activities. However it can be deployed in sediments and
provides profiling abilities to depths generally not achievable by other
methods. The probe consists of a tip and housing, the top of which is
sealed with a disc containing a flexible septum. The tip can be
constructed of porous high-density polyethylene (HDPE) that allows pore
water to enter the body when put under vacuum. The tip also can be
constructed of stainless steel. The stainless steel tip is driven to the
desired sampling depth and the body of the sampler is retracted to expose
a stainless steel screen that allows pore water to enter the sample
housing. A tool containing an evacuated sample vial (35 to 500 ml or 1.2
to 16.9 fluid ounces) with a septum cap and a double-ended hypodermic
needle is lowered down the push rod. When the tool encounters the
sample housing, the needle penetrates the housing septum at the same
time it penetrates the vial septum allowing pore water to enter the vial.
When the vial is full, the tool is retrieved, and the vial is stored for
subsequent analysis. The advantage of the porous HDPE filter tip is that
it yields a sample with low turbidity.
The push point sampler has a small diameter core
barrel with lance tip and a "T" type handle. The small
diameter barrel has holes drilled in the side at the
bottom to allow water to enter. A solid plastic rod is
placed in the barrel to prevent water and sediment
from entering the sampler during pushing. When the
sampling section of the barrel has been driven/pushed
to the target depth, the rod is withdrawn allowing pore
water to enter. The water is sampled using a
peristaltic pump or in some cases a syringe. This
system is useful primarily in shallow water although it
can be deployed by a diver.
The Navy's Trident probe (Figure 24 )is a direct push
system that provides depth specific temperature and conductivity data that can be used to
determine what depth water should be obtained from the water sampling probe. It can be
deployed by hand or diver. The on-board air hammer can drive the samplers into more
compacted or stiff sediments that would be difficult to achieve manually. One use of the tool is
better estimate where the groundwater/surface water interface is by looking at the differences in
temperature and conductivity of the surface water versus water at depth.
The diffusion gradients in thin films (DGT) technique is based on a simple device that
accumulates solutes on a binding agent after passage through a well defined diffusion layer. A
binding agent such as a resin, selective to the target ions in solution (e.g., Chelex for trace
metals), is immobilized in a thin layer of hydrogel (binding gel). It is separated from solution by
an ion permeable hydrogel layer (diffusive gel). Between the diffusive gel and the bulk solution
there is a diffusive boundary layer where transport of ions occurs solely by molecular diffusion.
Within a few minutes of deployment, a steady state linear concentration gradient is established
between the solution and the binding gel. By exploiting this simple steady state condition, the
DGT technique can measure fluxes in situ (Teasdale, 1999 and Davidson, et al., 2001).
 Figure 25. Benthic flux lander.
Figure 25. Benthic flux lander.
Courtesy: U. S. Navy
Benthic flux sampling devices (Figure 25) measure
the flux of analytes, natural and otherwise, at the
sediment surface water interface. This generally is
done by lander emplacement of a container open side
down in the sediment. The container is fabricated to
allow surface water to flow through it until the
sediment surface is penetrated where upon it seals
leaving the top portion of the container filled with
water. The water within the sealed box is
periodically stirred and sampled. Generally the
dissolved oxygen level of the trapped water is
monitored and the in situ level that existed when the
chamber was set is maintained. Tracers can also be
released to determine if there is a net loss from the
overlying water. Landers can operate unattended
from a few days to months depending upon their size
and design. They are fabricated to operate in shallow
or deep (6,000 m) environments. In addition to a
benthic flux chamber, some landers also can be
equipped with coring capabilities, advective flow
volume from the sediment to the surface water, and current measurement instrumentation.
Table 1. Typical Sample Volumes for Various Sediment Analyses.
| Sediment Analysis |
Minimum Sample Volume |
|---|
| Inorganic chemicals |
90 ml |
| Non-petroleum organic chemicals |
230 ml |
Other chemical parameters
(e.g., total organic carbon, moisture content) |
300 ml |
| Particle size |
230 ml |
| Petroleum hydrocarbons1 |
250-1000 ml |
| Acute and chronic whole sediment toxicity tests2 |
1-2 L |
| Bioaccumulation tests3 |
15 L |
| Benthic macroinvertebrate assessments |
8-16 L |
| Pore water extraction |
2 L |
| Elutriate preparation |
1 L |
1 The maximum volume (1,000 ml) is required only for oil and grease analysis; otherwise, 250 ml is sufficient.
2 Amount needed per whole sediment test (i.e., one species) assuming 8 replicates per sample and test volumes
specified in USEPA 2000.
3 Based on an average of 3 L of sediment per test chamber and 5 replicates (USEPA, 2000).
Table 2. Advantages and Disadvantages of Commonly Used Core Samplers.
| Device/
Dimensions |
Use |
Depth
of
Sample
(cm) |
Volume
of
Sample
(L) |
Advantages |
Disadvantages |
|---|
| Fluorocarbon
plastic or glass
tube (3.5-7.5
cm inner
diameter (ID);
≤ 120 cm long |
Shallow
wadeable waters
or deep waters if
SCUBA
available; soft or
semi-
consolidated
deposits |
0-10 |
1.1-5.3 |
- Preserves layering
and permits
historical study of
sediment
deposition
- Minimal risk of
contamination
- Rapid, samples
immediately ready
for laboratory
shipping
|
- Small sample size
necessitates repetitive
sampling
|
|
Hand corer
with
removable
fluorocarbon
plastic or glass
liners (3.5-7.5
cm ID; 120 cm
long) |
Same as above
except more
consolidated
sediments can be
obtained |
0-10 |
1.1-5.3 |
- Same advantages
as fluorocarbon
plastic or glass
tube
- Penetrates
substrate with
greater ease
through use of
handles
|
- Small sample size
requires repetitive
sampling
- Requires careful
handling to prevent
spillage
- Requires removal of
liners before repetitive
sampling
- Barrel and core cutter
metal may contaminate
sample
|
|
Box corer |
Same as above
but depth of
unconsolidated
sediment must be
at least 1 m |
0-70 |
≤ 30.0 |
- Collects large,
undisturbed
sample; optimal for
obtaining intact
subsamples
|
- Difficult to handle
- Relatively heavy;
requiring larger vessel
and power winch to
deploy
|
|
Gravity corer,
Phleger corer
(3.5 cm ID,
≤ 50 cm long) |
Deep lakes and
rivers; semi-consolidated
sediments |
0-50 |
≤ 0.48 |
- Reduces risk of
sample
contamination
- Maintains sediment
integrity relatively
well
- Penetrates with
sharp cutting edge
|
- Requires careful
handling to avoid
sediment spillage
- Requires repetitive and
time-consuming
operation and removal
of liners due to small
sample size
|
| Gravity corer,
Kajak-Brinkhurst
corer (5 cm
ID, ≤ 70 cm
long) |
Deep lakes and
rivers; soft fine
grained sediments |
0-70 |
≤ 1.37 |
- Collects greater
volume than the
Phleger corer
|
- Same as the Phleger
corer
|
|
Benthos
gravity corer
(6.6, 7.1 cm
ID, ≤ 3 m
long) |
Soft, fine-grained
sediments |
0-3 m |
≤ 10.26 |
- Retains complete
sample from tube
because the core
valve is fitted to
the core liner
- Fins promote
vertical penetration
|
- Requires weights for
deep penetration so
the required lifting
capacity is 750-1,000
kg
- Requires vertical
penetration
- Compacts sediment
sample
|
|
Alpine gravity
corer (3.5 cm
ID) |
Soft, fine-grained
semiconsolidated
substrates |
≤ 2 m |
≤ 1.92 |
- Allows different
penetration depths
due to
interchangeable
steel barrels
|
- Lacks stabilizing fins
for vertical penetration
- Requires lifting
capacity of 2,000 kg
- Disturbs sediment
strata and integrity
- Compacts sediment
sample
|
|
Large piston
corers |
Ocean floor and
deep lakes; most
substrates |
3-20 m |
5-40 |
- Typically recovers
a relatively
undisturbed
sediment core in
deep waters
|
- Requires lifting
capacity of 2,000 kg
- Piston and piston
positioning at
penetration may fail
- Disturbs surface (0-0.5
m) layer some
compaction possible
|
|
BMH-53
Piston corer |
Waters ≤ 2 m
deep with
extension rod;
soft deposits |
≤ 2 m |
≤ 2 |
- Piston provides for
greater sample
retention
|
- Metal barrels
introduce risk of metal
contamination
|
|
Boomerang
corer (6.7 cm
ID) |
Ocean floor |
1 m |
3.52 |
- Requires minimal
shipboard
equipment so
smaller vessels can
be used
|
- Only penetrates 1.2 m
- Requires calm water
for recovery
|
|
Ogeechee
(Stainless steel
2 inch ID 20-96 inch core
lengths) |
Waters up to 4.5
m with extension
rod; soft to firm
unconsolidated
material less than
0.5 mm in
diameter |
0.5-2.5m |
1-5 |
- Long core length
available
- Manual valve
tension adjustment
aids in sealing and
sample retention
|
- Stainless Steel
construction makes
longer core lengths
heavy.
|
|
Russian Peat
Corer (3
models core
length 20-40
inches; inside
diameter 2 or
3 inches) |
Sediments
amenable to
penetration by
slide hammering;
extension rods
allow for deep
sampling |
>15m |
0.5-1.45 |
- Light weight easy
to use
- Collects discrete
relatively
uncompressed/
undisturbed
samples
|
- Cover plate is exposed
to sediments to
sampling depth which
can result in cross
contamination.
- Gravels or debris can
hinder closing
|
|
Vibracorer
(5.0-7.5 cm
ID) |
Ocean and lakes;
and silty sand and
gravelly sand
substrates of any
water body |
3-6 m |
5.89-13.25 |
- For deep profiles it
effectively samples
most substrates
with minimum
disturbance
- Can be used in
over 20 m of water
depth
- Portable models
can be operated
from small vessels
|
- Labor intensive
- Assembly and
disassembly might
require divers
- Disturbs surface (0-0.5
m)
- Heavier models
require large boat and
power winch to deploy
- Core integrity slightly
disturbed
|
Adapted from Appendix E-2 USEPA, 2001.
Table 3. Advantages and Disadvantages of Commonly Used Grab Samplers
| Device |
Use |
Sample
Depth
(cm) |
Sample
Volume
(L) |
Advantages |
Disadvantages |
|---|
| Orange Peel |
Marine waters,
deep lakes |
0-18 |
10-20 |
- Comes in a range of
sizes
|
- Need large boat, powered
winch and cable line
- Blocking of jaws may
cause sample loss
|
|
Smith-McIntyre |
Deep lakes, rivers,
and estuaries |
0-20 |
10-20 |
- Trigger plates provide
added leverage
essential to penetrating
substrate
|
- Heavy, need boat and
power winch
- Inadequate for deep
burrowing organisms
|
|
Birge-Ekman,
small |
Lakes and marine
areas; soft
sediments, silt and
sand |
0-10 |
≤ 3.4 |
- Handles easily without
crane or winch
- Can be adapted for
shallow water use
- Good for soft
sediments, sand, and
silt
- Allows subsampling
|
- Restricted to low current
due to light weight and
messenger activation
- May exceed target
penetration depth
- Subsampling may be
restricted due to size of
top flaps
- Loss of fine surface
sediments may occur
during retrieval
|
|
Birge-Ekman,
large |
Lakes and marine
areas; soft
sediments, silt and
sand |
0-30 |
≤ 13.3 |
- Can be adapted for
shallow water use
- Good for soft
sediments, sand, and
silt
- Allows subsampling
|
- Restricted to low current
- May exceed target
penetration depth
- Heavy, requires winch
- Loss of fine surface
sediments may occur
during retrieval
|
|
PONAR, standard |
Deep lakes and
estuaries; useful
on sand, silt, or
clay |
0-10 |
7.25 |
- Most universal grab
sampler
- Adequate on most
substrates
- Large sample obtained
intact, permitting
subsampling
- Good for coarse and
firm bottom sediments
|
- May not close completely,
resulting in sample loss
- Heavy, requires winch
|
|
PONAR, petite |
Deep lakes, rivers,
and estuaries;
useful on sand,
silt, or clay |
0-10 |
1.0 |
- Adequate for most
substrates that are not
compacted
|
- May not penetrate
sediment to desired depth,
especially in consolidated
sediments
- Susceptible to incomplete
closure and loss of
sample
- Requires more casts to
obtain sufficient sample if
many analyses needed
|
|
Van Veen |
Deep lakes, rivers,
and estuaries;
useful on sand silt
or clay |
0-30 |
18-75 |
- Adequate on most
substrates that are not
compacted
- Large sample obtained
intact, permits
subsampling
- Available in stainless
steel
- Effective in marine
environments in deep
water and strong
currents
|
- May not close completely
resulting in sample loss
- May close prematurely in
rough waters
- Heavy, requires winch
|
|
Modified Van
Veen (e.g., "Ted-Young grab") |
Lakes and marine
areas |
0-15 |
≤ 18.0 |
- Fluorocarbon plastic
liner can help avoid
metal contamination
- Screened bucket cover
helps reduce bow wave
effects
|
- Requires winch
- Relatively expensive
|
|
Petersen |
Deep lakes, rivers,
and estuaries;
useful on most
substrates |
0-30 |
9.45 |
- Provides a large
sample
- Penetrates most
substrates
|
- Shock wave from descent
may disturb fine-grained
sediment
- Lacks lid cover to permit
subsampling
- May not close completely
- Restricted to low current
conditions
- May exceed target
penetration depth
|
|
Shipek, standard |
Marine waters and
large inland lakes
and reservoirs |
0-10 |
3.0 |
- Sample bucket opens
to permit subsampling
|
- Heavy, requires winch
- In some designs, can
result in the loss of the
topmost 2-3 cm of very
fine, unconsolidated
sediment
- Not useful for compacted
sandy clay or till
substrates
|
|
Mini Shipek |
Lakes |
0-3 |
0.5 |
- Handles easily without
winch or crane from
most platforms
- Useful for most
substrates that are soft
|
- Requires vertical
penetration
- Samples small volume
- In some designs, may lose
fine-grained sediment
- May close prematurely
|
Adapted from Appendix E-1 USEPA 2001.
References
The citations below are provided as additional references. The documents Methods for
Collection, Storage, and Manipulation of Sediments for Chemical and Toxicological Analyses
and Assessing Aquatic Ecosystems Using Pore Waters and Sediment Chemistry contain
extensive bibliographies on sediment sampling and testing.
Apitz, S., et al. 2002. Critical Issues for Contaminated Sediment Management, MESO-02-TM-01, Marine Environmental Support Office, U.S. Navy.
Burton, G. 1998. Assessing Aquatic Ecosystems Using Pore Waters and Sediment Chemistry.
Aquatic Effects Technology Evaluation Program, Natural Resources Canada.
California Environmental Protection Agency Department of Toxic Substances Control. 2000.
Certification of U.S. Navy Benthic Flux Sampling Device.
http://www.dtsc.ca.gov/TechnologyDevelopment/TechCert/company_index.cfm
Campbell, J., F. Lyford, and R. Willey. 2002. Comparison of Vapor Concentrations of Volatile
Organic Compounds with Ground-Water Concentrations of Selected Contaminants in Sediments
Beneath the Sudbury River, Ashland, Massachusetts, 2000. USGS Open-File Report 02-143.
Carr, R. and M. Nipper, (Eds). 2001. Summary of a SETAC technical workshop: Porewater
toxicity testing: biological, chemical, and ecological considerations with a review of methods
and applications, and recommendations for future areas of research. Summary of a SETAC
Technical Workshop: Porewater Toxicity Testing: Biological, Chemical, and Ecological
Considerations with a Review of Methods and Applications, and Recommendations for Future
Areas of Research; 18-22 March 2000, Pensacola, FL. Society of Environmental Toxicology and
Chemistry.
Chadwick, B. and A Hawkins. 2008. Monitoring of Water and Contaminant Migration at the Groundwater–Surface Water Interface, Final Cost and Performance Report, ER200422. SSC San Diego, Technical Report 1966, 75 pp.
http://www.spawar.navy.mil/sti/publications/pubs/tr/1966/tr1966cond.pdf
Chadwick, D., J. Groves, B. Harre, R. Paulsen, and C. Smith. 2003. Coastal Contaminant
Migration Monitoring: The Trident Probe and UltraSeep System: Hardware Description,
Protocols, and Procedures. SSC San Diego Technical Report 1902.
http://www.spawar.navy.mil/sti/publications/pubs/tr/1902/tr1902cond.pdf
Chandler, G. 2002. Assessment of Genetic, Population, and Community Level Effects of
Pesticides, PAHs, and Metal Mixtures on Sediment-Dwelling Meiobenthos.
http://enhs.sph.sc.edu/enhstc-res1.htm
Chapman, D. undated. The Virtual Fish:SPMD. USGS website:
http://wwwaux.cerc.cr.usgs.gov/SPMD/
Church, P.et al. 2002. Guidance on the Use of Passive-Vapor-Diffusion Samplers to Detect
Volatile Organic Compounds in Ground-Water-Discharge Areas, and Example Applications in
New England. USGS, Water-Resources Investigations Report 02-4186.
Davidson, W., et al. 2001. Dialysis, DET and DGT: In situ diffusional techniques for studying
water, sediments, and soils. In Situ Monitoring of Aquatic Systems: Chemical Analysis and
Speciation, J. Buffle and G. Horvai (Editors). John Wiley and Sons, Inc.
Euliss, N. and R. Barnes. 1992. A new device for collection of interstitial water from wetland
sediments. Wetlands Ecology and Management 1(4): pp. 233-237. Jamestown, ND: Northern
Prairie Wildlife Research Center Home Page.
http://www.npwrc.usgs.gov/resource/wetlands/newdevic/index.htm
Fones, G., et.al. 2001. High-resolution metal gradients measured by in situ DGT/DET
deployment in Black Sea sediments using an autonomous benthic lander. Limol. Oceanogr.
46(4), pp 982-988.
Hampton, T. and D. Chadwick. 2000. Quantifying In Situ Metal Contaminant Mobility in
Marine Sediments. San Diego Navy Space and Warfare Systems Center Technical Report 1826.
Holcombe, B., R. Keil, and A. Devol. 2001. Determination of pore water dissolved organic
carbon fluxes from Mexican margin sediments. Limnology and Oceanography, Vol. 46(2), pp
298-308.
Krom, M., et al. 2002. In-situ determination of dissolved iron production in recent marine
sediments. Aquatic Sciences 64 (2002) 282-291.
Lager, T., et al. 2002. Pore water sampling by means of dialysis technique and
centrifugation—predicting the source strength of harbour sludge. GeoProc2002, March 4-7,
2002, Bremen, Germany.
Mudroch, A. and J. Azcue. 1995. Manual of Aquatic Sediment Sampling. Lewis Publishers
Inc., Boca Raton, FL.
Mudroch, A. and S. MacKnight. (Eds). 1994. Handbook of Techniques for Aquatic Sediments
Sampling, Second Edition, ISBN: 1566700272. Lewis Publishers, Boca Raton, FL.
Navy Environmental Leadership Program. 1996. Petrex passive soil gas and sediment vapor
sampling system. NELP Fact Sheet No. 7.
New Jersey Department of Environmental Protection. 1999. Guidance for Sediment Quality
Evaluations. http://www.state.nj.us/dep/srp/regs/sediment/
Ohio Environmental Protection Agency. 2001. Sediment and Sampling Guide and
Methodologies (2nd Edition).
Radtke, D. 1997. Chapter A8. Bottom-material samples. National Field Manual for the
Collection of Water-Quality Data. USGS Book 9 Handbooks for Water-Resources
Investigations. http://water.usgs.gov/owq/FieldManual/Chapter8/index.html
Resources Inventory Committee. 1998. Lake and Stream Bottom Sediment Sampling Manual.
Province of British Columbia, Canada.
Schüürmann, B.V. 2000. Calibrating the uptake kinectics of semipermeable membrane devices
in water: the impact of hydrodynamics. 6th International SPMD Workshop and Symposium, July
25-27, 2000, USGS Columbia Environmental Research Center.
http://www.cerc.cr.usgs.gov/Brnch_Webs/EChem/workshop.htm
Shoven, H. 2000. Monitoring dioxin levels in Maine rivers with semipermeable membrane
devices. 6th International SPMD Workshop and Symposium, July 25-27, 2000, USGS Columbia
Environmental Research Center.
http://www.cerc.cr.usgs.gov/Brnch_Webs/EChem/workshop.htm
Society of Environmental Toxicology and Chemistry. 2001. Summary of a SETAC Technical
Workshop: Porewater Toxicity Testing: Biological, Chemical, and Ecological Considerations
with a Review of Methods and Applications, and Recommendations for Future Areas of
Research. http://www.setac.org/sites/default/files/PWSummary.pdf
South Carolina Department of Health and Environmental Control. 2001. Methods to Evaluate
Contaminated Groundwater Discharges to Surface Water.
Stüben, D. 2001. Development of a Pore Water Sampler (PWS) for Marine and Fresh Water
Use Up to 100 m Water Depth. Institute for Mineralogy and Geochemistry, University of
Karlsruhe, Karlsruhe Germany.
Teasdale, P. 1999. DGT (diffusive gradients in thin films), an in situ method of measuring
porewater concentrations or fluxes from solid to solution phase in sediments—background and
recent developments. Northwest Analytical Division of the Royal Chemical Society Workshop,
Liverpool (July, 1999).
Tetra Tech EM, Inc. 2003. Literature Review and Report: Surface Sediment Sampler Database.
Office of Research and Development, USEPA.
Tse, E., B. Richardson, and P. Lam. 2000. Uptake and Release of selected organochlorines and
PAHs by mussels and SPMDs. 6th International SPMD Workshop and Symposium, July 25-27,
2000, USGS Columbia Environmental Research Center.
http://www.cerc.cr.usgs.gov/Brnch_Webs/EChem/workshop.htm
USEPA, 2001. Methods for Collection, Storage, and Manipulation of Sediments for Chemical
and Toxicological Analyses: Technical Manual, EPA/823/B-01/002. Office of Water.
http://epa.gov/waterscience/cs/library/collection.html
USEPA. 2000. Methods for Measuring the Toxicity and Bioaccumulation of Sediment-associated
Contaminants with Freshwater Invertebrates. Second Edition. EPA/600/R-99/064, Duluth, MN.
USEPA. 1999. Innovative Technology Verification Report: Sediment Sampling Technology,
Aquatic Research Instruments Russian Peat Borer, EPA/600/R-01/010. Office of Research and
Development.
USEPA. 1998. Environmental Technology Verification Report: Soil Gas Sampling Technology
W. L. Gore & Associates, Inc. GORE-SORBER Screening Survey, EPA/600/R-98/095. Office of
Research and Development.
USEPA. 1994. Assessment Guidance Document, EPA/905/B-94/002. Assessment and
Remediation of Contaminated Sediments Program, Great Lakes National Program Office,
Chicago.
USEPA. 1987. Recommended Guidelines for Sampling Marine Sediment, Water Column, and
Tissue in Puget Sound. USEPA Region 10 Office of Puget Sound and Puget Sound Water
Quality Authority.
USEPA. 1986. Recommended Protocols for Measuring Conventional Sediment Variables in
Puget Sound. USEPA Region 10 Office of Puget Sound and Puget Sound Water Quality
Authority.
Winter, T. 2002. Subaqueous capping and natural recovery: Understanding the hydrogeologic
setting at contaminated sites. DOER Technical Notes Collection (TN-DOER-C26), U.S. Army
Engineer Research and Development Center, Vickburg, MS.
http://www.wes.army.mil/el/dots/doer
Winterhalter, B. 2000. The New Undisturbed Sandy Sediment Sampler. Geologic Survey of
Finland. http://www.kolumbus.fi/boris.winterhalter/OSCOR.pdf
Wisconsin Department of Natural Resources. 2001. 701.4 General sediment sampling
equipment and procedures. WI DNR Field Procedures Manual Internet Edition, Part B:
Collection Procedures.
http://www.dnr.state.wi.us/org/water/wm/wqs/sediment/sampling/701_4.htm




