Perchlorate
Chemistry and Behavior
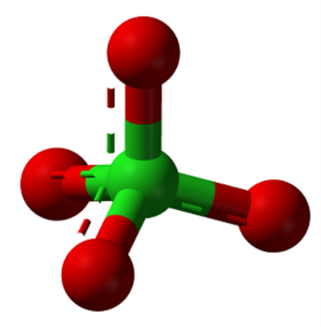
Perchlorate is an anion composed of four oxygen atoms and a chlorine atom (ClO4-)1 in a tetrahedral geometry (Figure 1). The chlorine atom has an oxidation state of +7, the most oxidized form of chlorine, which makes the molecule a strong oxidizing agent — hence its use as a primary component of rocket fuel (Brandenhuber, 2009; ITRC, 2005). Mixtures of a chlorate, such as potassium perchlorate, with charcoal, sugar, or another easily oxidized organic substance react explosively when heated or, in some circumstances, when rubbed together.
Despite its strength as an oxidizing agent, perchlorate has a high kinetic barrier that makes it very slow to react under normal environmental conditions (Urbansky, 2002). Perchlorate also has a low tendency to react with minerals in soil because of its negative charge (ATSDR, 2008). Its general lack of reactivity, low propensity to absorb to most soil surfaces, and high solubility in water make it very mobile. This can result in extensive groundwater plumes. For example, a perchlorate plume from the former Olin safety flare facility in California was found to extend 10 miles long (California EPA, 2016).
Perchlorate compounds do not volatilize from water or soil surfaces to air because of their low vapor pressures, but they can exist in air for a brief time as particulate matter. Rain or gravitational settling can cause perchlorate-containing particles to be transferred from the atmosphere to the ground surface. Soil particles contaminated with perchlorate can also be transported to the atmosphere by wind-borne erosion (ATSDR, 2008). Natural perchlorate can form via ozone and/or ultraviolet reactions with chloride in aerosols or sands, potentially catalyzed by an electrical discharge or lightning (Dasgupta et al. 2005).
References:
Agency for Toxic Substances and Disease Registry (ATSDR), 2008. ![]() Toxicological Profile for Perchlorates. U.S. Department of Health and Human Services. September.
Toxicological Profile for Perchlorates. U.S. Department of Health and Human Services. September.
Brandhuber, P. S. Clark and K. Morley, 2009. ![]() A Review of Perchlorate Occurrence in Public Drinking Water Systems. 11 pp.
A Review of Perchlorate Occurrence in Public Drinking Water Systems. 11 pp.
California EPA, 2016. ![]() State of California State Water Resources Control Board Order WQ 2005-0007 in the Matter of the Petitions of Olin Corporation and Standard Fusee, Incorporated for Review of Cleanup and Abatement Order No. R3-2004-0101 Issued by the California Regional Water Quality Control Board, Central Coast Region. SWRCB/OCC Files A-1654 and A-1654(a)
State of California State Water Resources Control Board Order WQ 2005-0007 in the Matter of the Petitions of Olin Corporation and Standard Fusee, Incorporated for Review of Cleanup and Abatement Order No. R3-2004-0101 Issued by the California Regional Water Quality Control Board, Central Coast Region. SWRCB/OCC Files A-1654 and A-1654(a)
Dasgupta, P.K., et al., 2005. The Origin of Naturally Occurring Perchlorate: The Role of Atmospheric Processes. Environmental Science and Technology. 39(6)1569-1575. Doi:10.1021/es048612x
Interstate Technology Regulatory Council (ITRC), 2005. ![]() Perchlorate: Overview of Issues, Status, and Remedial Options. PERC-1, 152 pp.
Perchlorate: Overview of Issues, Status, and Remedial Options. PERC-1, 152 pp.
Urbansky, E., 2002. ![]() Perchlorate as an Environmental Contaminant. Environmental Science & Pollution Research, 9(3) 187-192.
Perchlorate as an Environmental Contaminant. Environmental Science & Pollution Research, 9(3) 187-192.
Resources
Perchlorate: Environmental Problems and Solutions
Sellers, K., K. Weeks, W.R. Alsop, S.R. Clough, M. Hoyt, B. Pugh, and J. Robb. Taylor & Francis/CRC Press, Boca Raton, FL. ISBN: 0849380812, 226 pp, 2019
![]() Evaluation of the Potential for Monitored Natural Attenuation of Perchlorate in Groundwater
Evaluation of the Potential for Monitored Natural Attenuation of Perchlorate in Groundwater
Lieberman, M.T. and R.C. Borden. ESTCP Project ER-2004280428, 109 pp, 2010
Perchlorate (Anaerobic) Degradation Pathway Map
Gajjala, S. University of Minnesota, 2003
The Fate and Transport of Perchlorate in a Contaminated Site in the Las Vegas Valley. Part A: Investigation of the Influence of Biological Degradation and Sorption on the Fate of Perchlorate. Part B: Modeling of the Transport of Perchlorate in the Las Vegas Wash
Batista, J.R., D.S. James, P.S. Amy, B. Luke, P. Darrell, Y.-T. Chen, L. Papelis, and R. Unz, Univ. of Nevada – Las Vegas, EPA National Center for Environmental Research, EPA Grant Number R827622E03, 2001-2003
![]() Perchlorate as an Environmental Contaminant
Perchlorate as an Environmental Contaminant
Urbansky, E.
Environmental Science & Pollution Research 9(3) pp 187-192, 2002
![]() Perchlorate Chemistry: Implications for Analysis and Remediation
Perchlorate Chemistry: Implications for Analysis and Remediation
Urbansky, E. CRC Press, 1998
Helpful Information